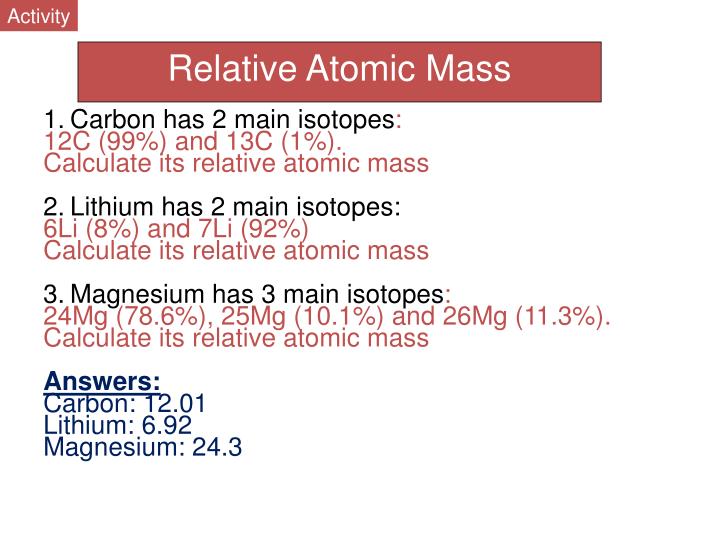
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 79Br | 78.918 338(7) | [0.505, 0.508] |
| 81Br | 80.916 288(6) | [0.492, 0.495] |
Bromine Mass Number
- ››More information on molar mass and molecular weight. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all.
- Bromine consists of two isotopes with masses of 78.92 and 80.92 amu. What is the abundances of these two isotopes?
- Agnes Parish, since its founding as a mission of St. Joseph Church, (the Cathedral of our Diocese of Baton Rouge), on May 27, 1912, on through its establishment as an independent parish on February 9, 1917, through the construction of the present church building in 1951, on up to the present day, has been a vibrant sign of the presence of Christ in His Church, in the People of God in south.
What Is Bromine
Molecular Formula Br 2; Average mass 159.808 Da; Monoisotopic mass 157.836655 Da; ChemSpider ID 22817. Mass Amount Half-Life Emissions (percent) (hr) (kg.
In 1961, the Commission recommended Ar(Br) = 79.909(2) based on a chemical determinationof the mass ratio AgBr/Ag and an updated evaluation of the atomic weight of silver, which was then the major source of the uncertainty in Ar(Br). The values of Ar(Br) and Ar(Ag)were especially important at that time because the atomic weights of many other elements were determinedby the mass ratios of their bromides to Ag or AgBr. In its 1965 re-evaluation, summarized in the1967 report, the Commission adopted the recent mass-spectrometric measurements, which yielded Ar(Br) = 79.904(1) and essentially ended the era of chemical determinations ofatomic weights for this element.

The standard atomic weight of bromine was last revised in 2011 to better reflect the natural variations of Ar(Br) in natural materials.

Atomic weights of the elements 2011 by M.E. Wieser et al. Pure Appl. Chem. 2013 (85) 1047-1078
CIAAW
Bromine
Ar(Br) = [79.901, 79.907] since 2011
The name derives from the Greek bromos for 'bad stench' or 'bad odour'. It was first prepared by theGerman chemist Carl Löwig in 1825, but it was first publicly announced in 1826 by the French chemistand pharmacist Antoine-Jérôme Balard, and so the discovery is, therefore, credited to him.
Natural variations of bromine isotopic composition
Bromine Mass
Isotopic reference materials of bromine.
